1剂接种,快速安心
通用名称:重组新型冠状病毒疫苗(5型腺病毒载体) 商品名称:克威莎(Convidecia) 英文名称:Recombinant COVID-19 Vaccine(Adenovirus Type 5 Vector)
康希诺生物新冠疫苗研发历程
-
2021-12公开发布Ⅲ期临床试验最终有效率和期中安全性分析结果
-
2021-09助力马来西亚建设本地灌装生产线
-
2021-07全球首个吸入式新冠疫苗临床I期试验结果在柳叶刀发表
-
2021-06巴基斯坦发布本地灌装生产的康希诺新冠疫苗
-
2021-05-21获得欧盟GMP证书
-
2021-04获得智利紧急使用授权
-
2021-03-22吸入用重组新型冠状病毒疫苗获批进入临床
-
2021-03获得匈牙利紧急使用授权
-
2021-03康希诺生物同墨西哥地方企业合作建设的灌装生产线
-
2021-02-25获得国家药监局批准附条件上市
-
2021-02-12获得巴基斯坦紧急使用授权
-
2021-02-10获得墨西哥紧急使用授权
-
2021-02-01中期数据达到预设安全性及有效性标准
-
2020-09启动全球多中心III期临床试验
-
2020-07-20全球率先发布II期临床试验结果
-
2020-06-25获得军队特需药品批件
-
2020-06-14获得新冠疫苗生产许可证
-
2020-05-22全球率先发布I期临床试验结果
-
2020-04-12全球率先启动II期临床
-
2020-03-16全球率先启动I期临床
-
2020-01-20新冠项目立项
克威莎

克威莎是康希诺生物股份公司和中国军事科学院军事医学研究院生物工程研究所陈薇院士团队研发生产的重组新型冠状病毒疫苗(5型腺病毒载体),2021年2月25日获得国家药品监督管理局批准附条件上市。
主要特点为:
1剂,14天快速保护

14天总体保护率为68.83%,重症保护率为95.47%
28天总体保护率为65.28%,重症保护率为90.07%
1剂,激发体液免疫和细胞免疫,提供双重保护
1剂,成本效益更优

减少接种费用及工作量
提高接种依从性
克威莎新闻
新冠病毒和新冠疫情
新冠病毒
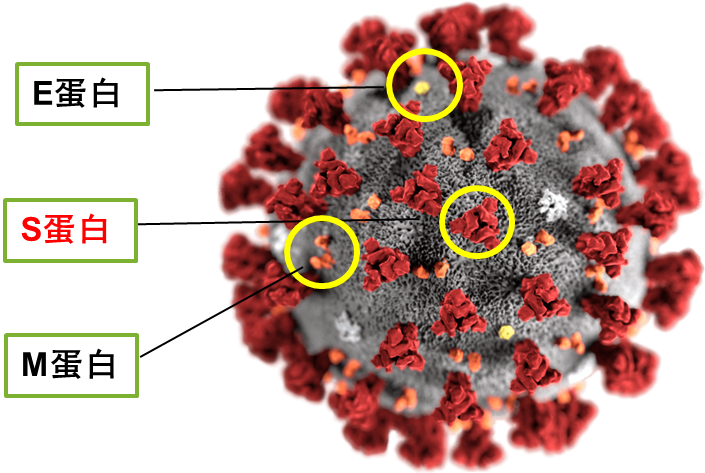
新型冠状病毒(SARS-CoV-2)是一种具有包膜的正链单股RNA病毒,属于冠状病毒科乙型冠状病毒属严重急性呼吸道综合征相关冠状病毒种。SARS-CoV-2病毒可通过人类上呼吸道入侵人体,以多种细胞表面表达的ACE2为受体达到感染[1]。
早期研究证明针对新冠病毒S蛋白的抗体可有效中和病毒并预防感染,是中和抗体和疫苗设计的主要靶标[2]。
2019新冠病毒 Centers for Disease Control and Prevention/AFP/File
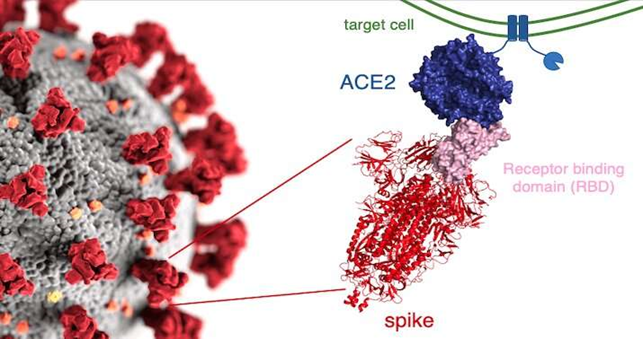
新冠病毒通过S蛋白结合到宿主细胞表面的ACE2受体上从而感染宿主/ A New Map Catalogs the Effects of Coronavirus Mutations
新冠疫情
新型冠状病毒肺炎(新冠肺炎,COVID-19)为新发急性呼吸道传染病,目前已成为全球性重大的公共卫生事件。通过积极防控和救治,我国境内疫情基本得到控制,仅在个别地区出现局部暴发和少数境外输入病例。由于全球疫情仍在蔓延,且有可能较长时期存在,在建立起群体免疫屏障前,新冠肺炎在我国仍存在传播和扩散的风险。
腺病毒载体技术详解
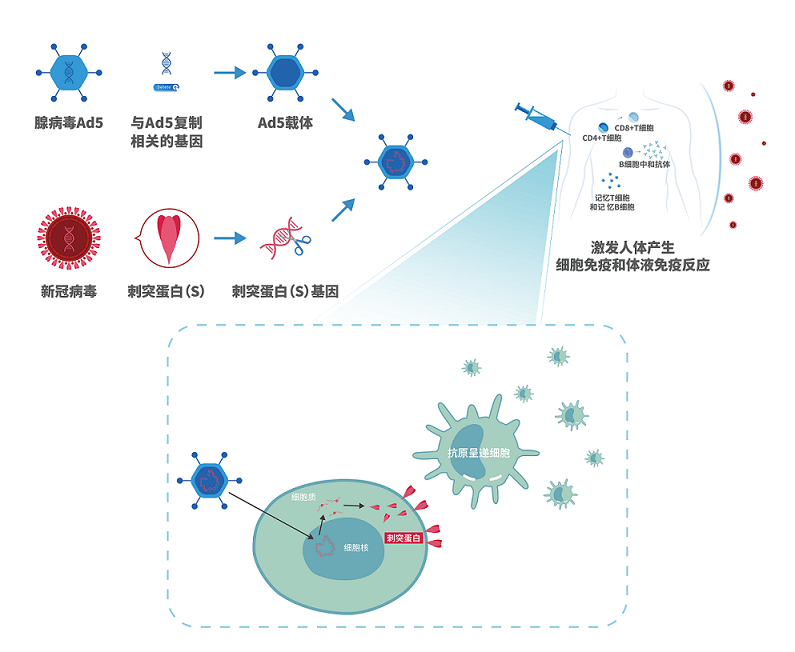
• 作为载体的人5型腺病毒剔除了复制相关基因,在人体内不会复制;
• 在被剔除基因的位置上,插入新冠病毒刺突蛋白(S蛋白)的基因;
• 腺病毒载体携带S基因进入人体细胞;
• S基因进入人体细胞后合成S蛋白,作为抗原激发机体产生免疫应答。
全球多中心临床试验
该新冠疫苗已经在2020年9月全面开展全球多中心三期临床试验。克威莎的III期临床试验为一项全球多中心、随机、双盲、安慰剂对照、适应性试验,旨在评估克威莎在18岁及以上成年人中的安全性、免疫原性和有效性。所有受试者在第0天接种1剂疫苗或安慰剂,并在为期52周的时间内跟踪监测疫苗的有效性及严重不良反应(SAE)。克威莎的临床研究的主要有效性目的为:评价疫苗接种后28天至52周内预防COVID-19疾病的保护效力,COVID-19疾病病例指所有出现COVID-19症状且经聚合酶链式反应(PCR)确认为阳性的病例(不区分症状的严重程度)。将在试验组和对照组间比较COVID-19的发病率。主要安全性目的为:评价所有受试者接种后52周内的SAE和需医疗干预的不良事件的发生率。
克威莎的III期临床试验已在三个大洲中的巴基斯坦、墨西哥、俄罗斯、智利、阿根廷,共78家临床研究中心,完成对4万余受试者的接种,该试验由来自七个国家的全球主要研究者(global PI)、全球协同主要研究者(global co-PI) 及各国协同主要研究者(country co-PI)共同领导,并遵循严格的伦理标准及严谨的科学准则。
常见问题
-
问1 请问新冠疫苗使用的腺病毒载体技术的原理是什么?答 作为载体的人5型腺病毒(Ad5)剔除了复制相关基因,在人体内不会复制;在剔除的那段基因的位置上插入了另一段基因,插入的这段基因是用于合成新冠病毒刺突蛋白(S蛋白)的;腺病毒载体相当于火箭,基因相当于卫星,载体携带基因进入人体细胞;基因进入人体细胞后合成S蛋白,S蛋白作为抗原激发机体的免疫应答。
-
问2 请问接种新冠疫苗后可否不再佩戴口罩?答 在人群疫苗接种达到较高免疫保护水平之前,广大群众仍然要保持预防新冠感染和传播的意识,做好个人卫生习惯,无论是否接种疫苗,在人群聚集的室内或封闭的场所仍然需要继续佩戴口罩,并遵循各地具体的防控措施要求。
-
问3 请问接种新冠疫苗前后有哪些注意事项?答 ① 接种前,接种医生会询问健康状况,请如实告知相关信息。 ② 接种完成后,需现场留观30分钟,接种当日注射部位保持干燥并注意个人卫生,适当安排休息。 ③ 接种后一周内避免接触个人既往已知过敏物及常见致敏原,尽量不饮酒、不进食辛辣刺激或海鲜类食物,建议清淡饮食、多喝水。
-
问4 请问新冠疫苗接种禁忌和暂缓接种人群包括什么?答 新冠疫苗的接种禁忌,必须严格遵守新冠疫苗说明书的规定。通常,对疫苗或疫苗中任何一种成分严重过敏者是接种禁忌对象。对于发热、急性疾病患者及慢性疾病急性发作者,一般属于暂缓接种对象。
-
问5 公司为什么会选择使用腺病毒载体技术开发新冠疫苗?答 疫情之初我公司管理层和研发人员在评估疫情严重程度以及疫苗开发紧迫性的同时,也在布局新冠疫苗研发的技术路线。由于疫情的紧迫性,病毒载体、mRNA等能够在相对短时间内研发成功的技术路线都在评估范围之内,我们希望能够在最短的时间内研制出安全有效的疫苗。经评估,我们优先选择了腺病毒载体技术来研制新冠疫苗,我公司拥有成熟的腺病毒载体技术平台,以此平台为依托,我们开发了肺结核加强疫苗、带状疱疹疫苗、寨卡病毒疫苗和重组埃博拉病毒病疫苗,其中,与军科院陈薇院士团队联合研发的重组埃博拉病毒病疫苗于2017年获得新药证书和注册批件。正是基于现有成熟的技术平台和科研专家,我公司新冠疫苗的研发非常迅速,2020年1月20日立项,3月16日启动一期临床试验,是全球首个启动一期临床试验的新冠疫苗。
-
问6 备孕女性、癌症患者、有慢性病的市民是否可以接种?答 近期怀孕的女性不建议接种疫苗,备孕女性建议接种后,要留出产生抗体的足够时间。另外,新冠疫苗说明书中妊娠期和哺乳期妇女暂缓接种。有糖尿病、高血压等基础病的市民如果通过药物可以控制,稳定期内可以接种疫苗。对于癌症患者来说,要咨询自己的肿瘤医生并结合用药情况判定是否可以接种。目前,患有神经系统疾病的人群不适合接种。
-
问7 春季到来过敏人群增多,处在过敏期的人群是否可以接种疫苗?答 过敏是医生在接种时考虑的一个重要因素,对疫苗或疫苗成分过敏,既往发生过疫苗严重过敏反应的人群不适合接种。对于花粉过敏、鼻塞、流鼻涕或者出现皮疹的过敏人群,建议要错开过敏期,轻症可遵医嘱进行接种。
相关链接和资源






