
-
2022年
 重组新型冠状病毒疫苗克威莎®序贯加强在中国获批、被列入世卫组织紧急使用清单; 全球首个吸入用新冠疫苗克威莎®雾优®在中国及摩洛哥获批紧急使用; 上海研发中心、mRNA产业化基地建成
重组新型冠状病毒疫苗克威莎®序贯加强在中国获批、被列入世卫组织紧急使用清单; 全球首个吸入用新冠疫苗克威莎®雾优®在中国及摩洛哥获批紧急使用; 上海研发中心、mRNA产业化基地建成 -
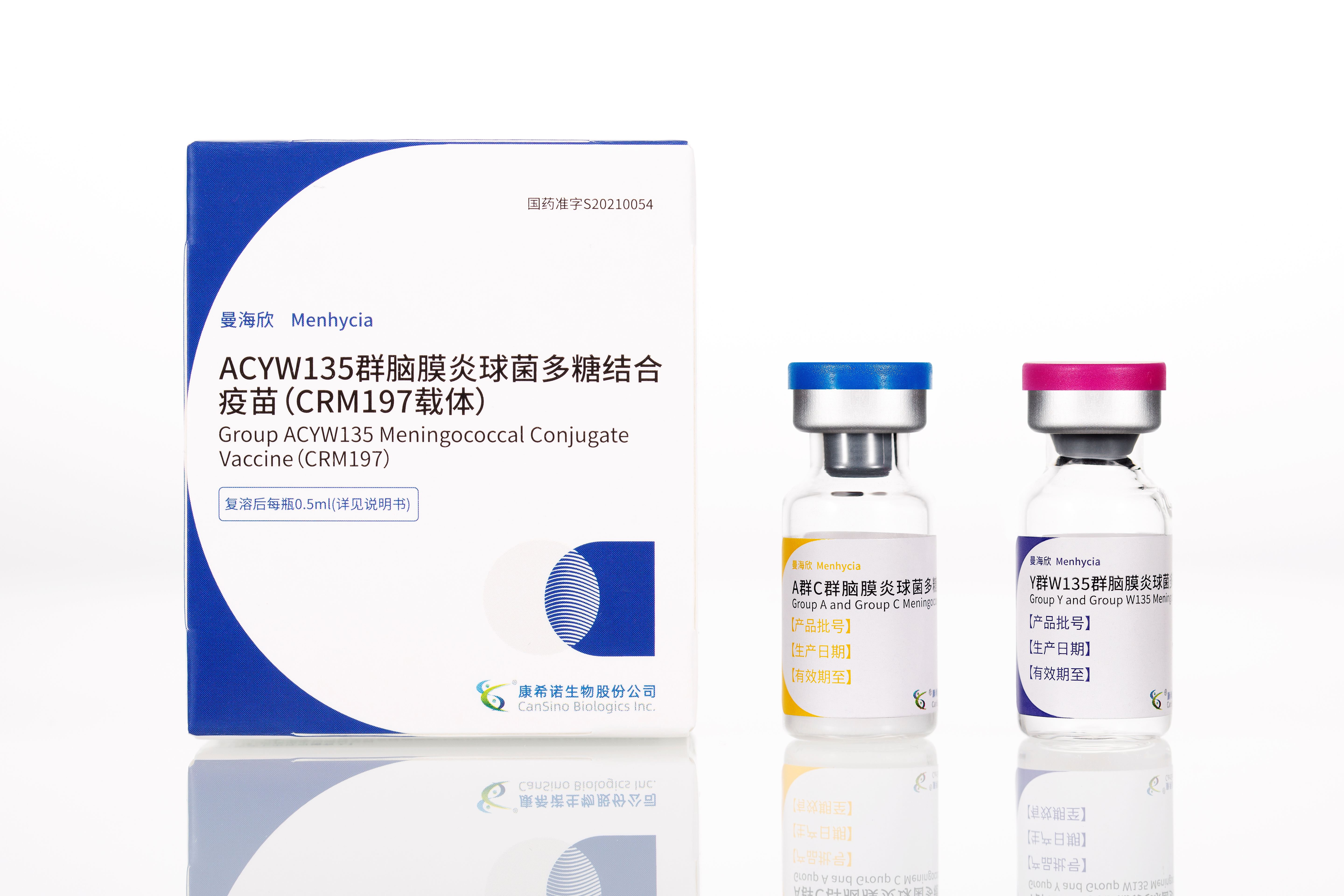
2021年重组新型冠状病毒疫苗(5型腺病毒载体)、MCV2及MCV4获批上市
-
2020年
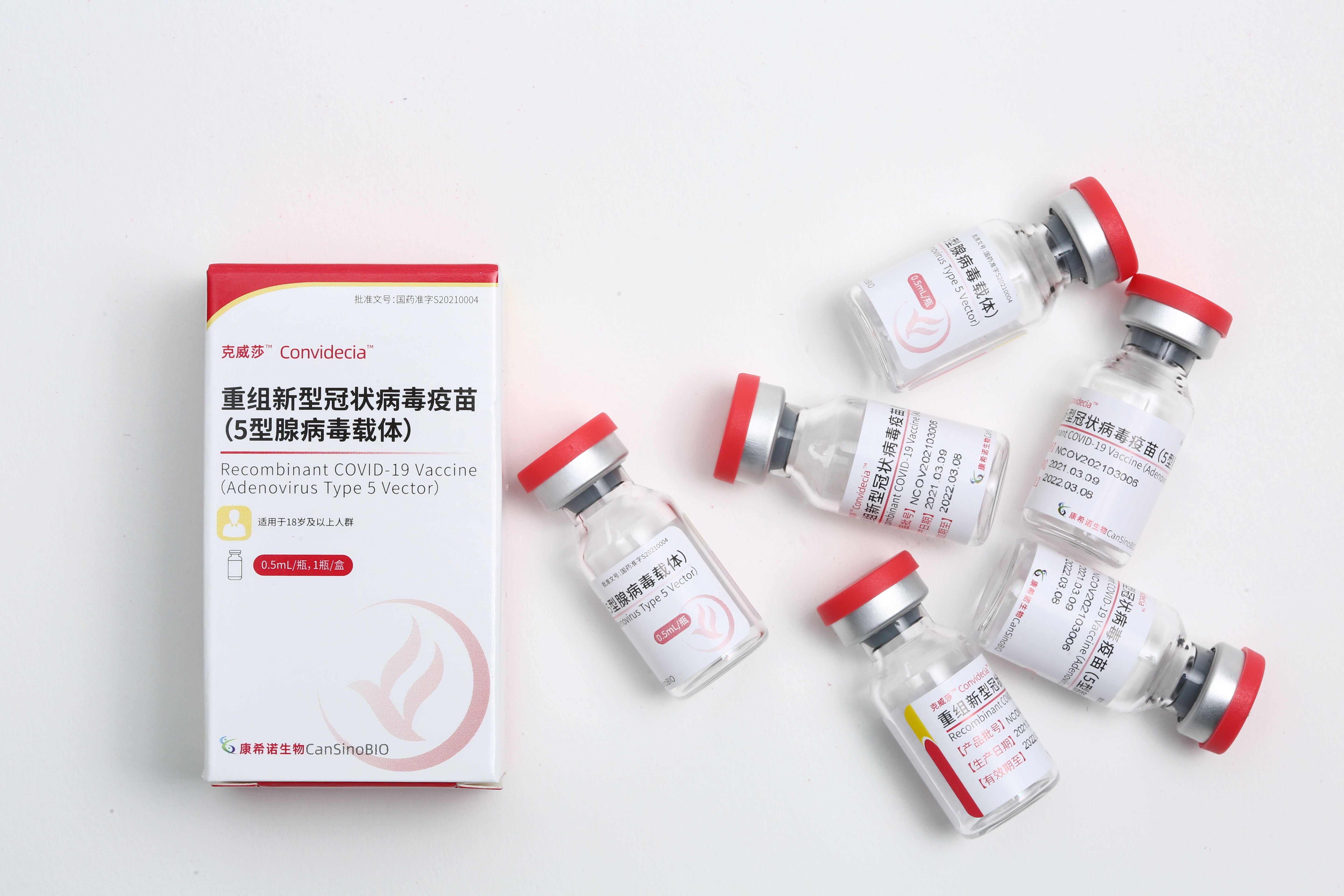 研发重组新型冠状病毒疫苗(腺病毒载体);A股发行获批成功挂牌上市
研发重组新型冠状病毒疫苗(腺病毒载体);A股发行获批成功挂牌上市 -
2019年MCV2获得新药注册申请受理;
香港主板上市;
MCV4新药注册申请获得优先审评
-
2018年
 完成商业生产中心的建设;
完成商业生产中心的建设;
DTcP及PBPV获得临床试验申请批准;
完成MCV2及MCV4的Ⅲ期临床试验;
PCV13i提交临床试验申请; -
2017年于中国取得Ad5-EBOV的新药申请批准
-
2016年
 Ad5-EBOV于塞拉利昂完成第二阶段临床试验;
Ad5-EBOV于塞拉利昂完成第二阶段临床试验;
PBPV提交临床试验申请 -
2015年中试车间通过EMA的QP认证;
MCV2及MCV4获得临床试验申请批准 -
2014年就在研DTcp提交临床试验申请;
AD5-EBOV获得临床试验申请批准并开始临床试验 -
2013年就MCV2及MCV4提交临床试验申请
-
2012年完成设计符合GMP标准的中试车间的建设
-
2009年于中国天津注册成立及登记







